[amazon_link asins=’1496326156,1455740969,0781753228,1493903411,1416033432,144191689X,1841843164,0062418173,0967038448′ template=’ProductCarousel’ store=’finmeacur-20′ marketplace=’US’ link_id=’11a01150-0970-11e8-81ed-39a60e701c07′]
Alternative Names:Renal transplant; Transplant – kidney
Definition:
A kidney transplant is surgery to place a healthy kidney into a person with kidney failure. Kidney transplantation or renal transplantation is the organ transplant of a kidney in a patient with end-stage renal disease. Kidney transplantation is typically classified as deceased-donor (formerly known as cadaveric) or living-donor transplantation depending on the source of the recipient organ. Living-donor renal transplants are further characterized as genetically related (living-related) or non-related (living-unrelated) transplants, depending on whether a biological relationship exists between the donor and recipient.
..CLICK & SEE THE PICTURES
Description :
Kidney transplants are one of the most common transplant operations in the United States.
A donated kidney is needed to perform a kidney transplant.
The donated kidney may be from:
*Living related donor — related to the recipient, such as a parent, sibling, or child
*Living unrelated donor — such as a friend or spouse
Indications:
The indication for kidney transplantation is end-stage renal disease (ESRD), regardless of the primary cause. This is defined as a drop in the glomerular filtration rate (GFR) to 20-25% of normal. Common diseases leading to ESRD include malignant hypertension, infections, diabetes mellitus and glomerulonephritis; genetic causes include polycystic kidney disease as well as a number of inborn errors of metabolism as well as autoimmune conditions including lupus and Goodpasture’s syndrome. Diabetes is the most common cause of kidney transplant, accounting for approximately 25% of those in the US. The majority of renal transplant recipients are on some form of dialysis – hemodialysis, peritoneal dialysis, or the similar process of hemofiltration – at the time of transplantation. However, individuals with chronic renal failure who have a living donor available often elect to undergo transplantation before dialysis is needed.
Sources of kidneys:
Since medication to prevent rejection is so effective, donors need not be genetically similar to their recipient. Most donated kidneys come from deceased donors, with some coming from living donors. However, the utilization of living donors in the United States is on the rise. In the year 2006, 47% of donated kidneys were actually from living donors (Organ Procurement and Transplantation Network, 2007). It is important to note that this varies by country: for example, only 3% of transplanted kidneys during 2006 in Spain came from living donors (Organización Nacional de Transplantes (ONT), 2007).
Living donors:
Potential donors are carefully evaluated on medical and psychological grounds. This ensures that the donor is fit for surgery and has no kidney disease whilst confirming that the donor is purely altruistic. Traditionally, the donor procedure has been through a single, 4-7 inch incision but live donation is being increasingly performed by laparoscopic surgery. This reduces pain and accelerates recovery for the donor. Excellent results have been demonstrated with laparoscopic donor nephrectomy, for both donor and recipient outcomes. Overall, recipients of kidneys from live donors do extremely well, in comparison to deceased donor recipients.
In 2004 the FDA approved the Cedars-Sinai High Dose IVIG therapy which reduces the need for the living donor to be the same blood type (ABO compatible) or even a tissue match. The therapy reduced the incidence of the recipient’s immune system rejecting the donated kidney in highly-sensitized patients
PROCEDURE FOR A LIVING KIDNEY DONOR:-
If you are donating a kidney, you will be placed under general anesthesia before surgery. This means you will be asleep and pain-free. The surgeon makes a cut in the side of your abdomen, removes the proper kidney, and then closes the wound. The procedure used to require a long surgical cut. However, today surgeons can use a short surgical cut (mini-nephrectomy) or laparoscopic techniques.
Deceased donors:-
Deceased donors can be divided in two groups:
Brain-dead (BD) donors
Donation after Cardiac Death (DCD) donors
Although brain-dead (or “heart-beating”) donors are considered dead, the donor’s heart continues to pump and maintain the circulation. This makes it possible for surgeons to start operating while the organs are still being perfused. During the operation, the aorta will be cannulated, after which the donor’s blood will be replaced by an ice-cold storage solution, such as UW (Viaspan), HTK, or Perfadex. [Depending on which organs are transplanted, more than one solution may be used simultaneously.] Due to the temperature of the solution (and since large amounts of cold NaCl-solution are poured over the organs for a rapid cooling of the organs), the heart will stop pumping.
“Donation after Cardiac Death” donors are patients who do not meet the brain-dead criteria, but due to the small chance of recovery have elected, via a living will or through family, to withdraw support. In this procedure, treatment is discontinued (mechanical ventilation is shut off). Usually, a certain amount of minutes after death has been pronounced, the patient is rushed to the operating theatre, where the organs are recovered, after which the storage solution is flushed through the organs itself. Since the blood is no longer being circulated, coagulation must be prevented with relatively large amounts of anti-coagulation agents, such as heparin. It is important to note that several ethical and procedural guidelines must be followed, chief of which is that the organ recovery team should not participate in the patient’s care in any manner until after death has been declared.
Kidneys from brain-dead donors are generally of a superior quality, since they have not been exposed to warm ischemia (the time between the heart stopping and the kidney being cooled).
Compatibility:
If plasmapheresis or IVIG is not performed, the donor and recipient have to be ABO blood group compatible. Also, they should ideally share as many HLA and “minor antigens” as possible. This decreases the risk of transplant rejection and the need for another transplant. The risk of rejection may be further reduced if the recipient is not already sensitized to potential donor HLA antigens, and if immunosuppressant levels are kept in an appropriate range. In the United States, up to 17% of all deceased donor kidney transplants have no HLA mismatch. However, it is important to note that HLA matching is a relatively minor predictor of transplant outcomes. In fact, living non-related donors are now almost as common as living (genetically)-related donors.
In the 1980s, experimental protocols were developed for ABO-incompatible transplants using increased immunosuppression and plasmapheresis. Through the 1990s these techniques were improved and an important study of long-term outcomes in Japan was published. . Now, a number of programs around the world are routinely performing ABO-incompatible transplants.
In 2004 the FDA approved the Cedars-Sinai High Dose IVIG protocol which eliminates the need for the donor to be the same blood type.
Procedure:
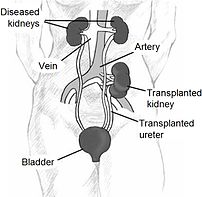
Since in most cases the barely functioning existing kidneys are not removed because this has been shown to increase the rates of surgical morbidities, the kidney is usually placed in a location different from the original kidney (often in the iliac fossa), and as a result it is often necessary to use a different blood supply:
*The renal artery of the kidney, previously branching from the abdominal aorta in the donor, is often connected to the external iliac artery in the recipient.
*The renal vein of the new kidney, previously draining to the inferior vena cava in the donor, is often connected to the external iliac vein in the recipient.
Why the Procedure is Performed :
A kidney transplant may be recommended if you have kidney failure caused by:
*Diabetes
*Glomerulonephritis
*Severe, uncontrollable high blood pressure
*Certain infections
A kidney transplant alone may NOT be recommended if you have:
*Certain infections, such as TB or osteomyelitis
*Difficulty taking medications several times each day for the rest of your life
*Heart, lung, or liver disease
*Other life-threatening diseases
Risks Factor:
The risks for any anesthesia are:
*Problems breathing
*Reactions to medications
The risks for any surgery are:
*Bleeding
*Infection
Other risks include:
Infection due to medications that suppress the immune response that must be taken to prevent transplant rejections
Post operation:
The transplant surgery lasts about three hours. The donor kidney will be placed in the lower abdomen and its blood vessels connected to arteries and veins in the recipient’s body. When this is complete, blood will be allowed to flow through the kidney again, so the ischemia time is minimized. In most cases, the kidney will soon start producing urine. Since urine is sterile, this has no effect on the operation. The final step is connecting the ureter from the donor kidney to the bladder.
Depending on its quality, the new kidney usually begins functioning immediately. Living donor kidneys normally require 3-5 days to reach normal functioning levels, while cadaveric donations stretch that interval to 7-15 days. Hospital stay is typically for four to seven days. If complications arise, additional medicines may be administered to help the kidney produce urine.
Medicines are used to suppress the immune system from rejecting the donor kidney. These medicines must be taken for the rest of the patient’s life. The most common medication regimen today is : tacrolimus, mycophenolate, and prednisone. Some patients may instead take cyclosporine, rapamycin, or azathioprine. Cyclosporine, considered a breakthrough immunosuppressive when first discovered in the 1980’s, ironically causes nephrotoxicity and can result in iatrogenic damage to the newly transplanted kidney. Blood levels must be monitored closely and if the patient seems to have a declining renal function, a biopsy may be necessary to determine if this is due to rejection or cyclosporine intoxication.
Acute rejection occurs in 10% to 25% of people after transplant during the first sixty days. Rejection does not necessarily mean loss of the organ, but may require additional treatment.
Complications:
Problems after a transplant may include:
*Transplant rejection (hyperacute, acute or chronic)
*Infections and sepsis due to the immunosuppressant drugs that are required to decrease risk of rejection
*Post-transplant lymphoproliferative disorder (a form of lymphoma due to the immune suppressants)
*Imbalances in electrolytes including calcium and phosphate which can lead to bone problems amongst other things
*Other side effects of medications including gastrointestinal inflammation and ulceration of the stomach and esophagus, hirsutism (excessive hair growth in a male-pattern distribution), hair loss, obesity, acne, diabetes mellitus (type 2), hypercholesterolemia, and others.
*The average lifetime for a donor kidney is ten to fifteen years. When a transplant fails a patient may opt for a second transplant, and may have to return to dialysis for some intermediary time.
Prognosis:
Kidney transplantation is a life-extending procedure. The typical patient will live ten to fifteen years longer with a kidney transplant than if kept on dialysis. The years of life gained is greater for younger patients, but even 75 year-old recipients (the oldest group for which there is data) gain an average four more years’ life. People generally have more energy, a less restricted diet, and fewer complications with a kidney transplant than if they stay on conventional dialysis.
Some studies seem to suggest that the longer a patient is on dialysis before the transplant, the less time the kidney will last. It is not clear why this occurs, but it underscores the need for rapid referral to a transplant program. Ideally, a kidney transplant should be pre-emptive, i.e. take place before the patient starts on dialysis.
At least three professional athletes have made a comeback to their sport after receiving a transplant: NBA players Sean Elliott and Alonzo Mourning; and New Zealand rugby union player Jonah Lomu as well as the German-Croatian Soccer Player Ivan Klasni?.
Recovery
The recovery period is 4 – 6 weeks for people who donate a kidney. If you’ve done so, you should avoid heavy activity during this time. Your doctor removes the stitches after a week or so.
If you received a donated kidney, you will need to stay in the hospital for about a week. Afterwards, you will need close follow-up by a doctor and regular blood tests.
Resources:
http://en.wikipedia.org/wiki/Kidney_transplantation
http://www.nlm.nih.gov/medlineplus/ency/article/003005.htm
![Reblog this post [with Zemanta]](https://i0.wp.com/img.zemanta.com/reblog_e.png?w=580)