[amazon_link asins=’B01J8WALE0,B00GNOPG8S,1331951720,1893441776,B01MXOPZIB,B01J2IC12U,B01J37411E,B009KT2GWM,B01J2GLUVU’ template=’ProductCarousel’ store=’finmeacur-20′ marketplace=’US’ link_id=’316e2df4-5fab-11e7-aab1-4db380a805de’]
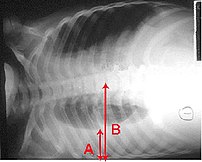
Definition:— Thoracentesis is a procedure used to obtain a sample of fluid from the space around the lungs. Normally, only a thin layer of fluid is present in the area between the lungs and chest wall (show radiograph 1). However, some conditions can cause a large amount of fluid to accumulate. This collection of fluid is called a pleural effusion (show radiograph 2). Thoracentesis is done to collect a sample of the fluid, which can help determine why the pleural effusion developed.
CLICK & SEE
Some infections and diseases cause fluid to accumulate in the space between the lung and the rib cage or between the lung and the diaphragm. This collection of fluid is called a pleural effusion. A pleural effusion might be detected on a chest x-ray. Sampling this fluid is important because it enables doctors to understand what caused the fluid to collect and how to treat the problem. The fluid can be sampled with a needle.
Reasons for Thoracentesis: — A thoracentesis is performed to determine the cause of a pleural effusion. In some cases, a physician may perform thoracentesis to relieve symptoms caused by the pleural effusion, including shortness of breath and low blood oxygen levels. A pleural effusion may be detected during a physical examination or on a chest x-ray.
Pleural effusion can be caused by many different conditions, including infections, heart failure, cancer, or tuberculosis. In some cases, blood or other fluid may be leaking into the pleural space from another part of the body, causing the effusion. By examining the fluid and the types of cells it contains, the cause of the effusion can usually be determined.
In general, there is no reason a thoracentesis cannot be performed. The procedure is more easily performed and complications are fewer when the pleural effusion is large. Special consideration may be necessary in patients who are on respirators.
Patients who have a bleeding disorder or who are on medications that affect blood clotting may need extra care to minimize the risk of bleeding. Patients should tell their healthcare provider if they have a history of bleeding problem or if they are taking medicine that decreases blood clotting. In some cases, a blood test will be taken prior to the procedure to exclude any blood clotting abnormalities caused by disease or medications.
Procedure: — A thoracentesis involves the following steps:
*The patient will be placed in a position that allows the doctor to easily access the effusion. Usually, the patient is asked to sit upright during the procedure. It is important to remain still during the procedure so that the fluid does not shift.
*The skin is cleaned with an antibacterial solution in the area where the needle will be inserted.
*A small amount of numbing medicine (a local anesthetic, similar to novocaine) is injected into the area. This medicine helps minimize discomfort during the procedure.
*A slightly larger needle is inserted in the same location. A syringe is attached to this needle and is used to withdraw fluid from around the lung. Patients who have symptoms from the effusion (eg, shortness of breath) may have a large amount of fluid removed, which allows the lung to re-expand.
*The needle is removed and pressure is briefly applied to the insertion site.
How do you prepare for the test?
You will need to sign a consent form giving your doctor permission to perform this test. Some patients have this test done in a doctor’s office, while others are admitted to the hospital for it. Generally your doctor will decide whether you need to be in the hospital based on your medical condition. A chest x-ray or an ultrasound is done before the procedure.
Tell your doctor if you have ever had an allergic reaction to lidocaine or the numbing medicine used at the dentist’s office. If you take aspirin, nonsteroidal anti-inflammatory drugs, or other medicines that affect blood clotting, talk with your doctor. It may be necessary to stop or adjust the dose of these medicines before your test.
What happens when the test is performed?
You wear a hospital gown and sit on a bed or table leaning forward against some pillows. The doctor listens to your lungs with a stethoscope and may tap on your back to find out how much fluid has collected.
Soap is used to disinfect an area of skin on one side of your back. A small needle is used to numb a patch of skin between two of your lower ribs. The numbing medicine usually stings for a second. A needle on an empty syringe is then inserted into the fluid pocket. Usually this pocket is around one inch below the skin surface. You might feel some minor pressure as the needle is inserted. Depending on the quantity of fluid that the doctor plans to remove, either the syringe itself is filled or soft plastic tubing is used to remove fluid into a collection bag or jar. While the doctor is attaching the tubing, he or she might ask you to hum out loud. This humming is for your safety: It prevents you from taking a deep breath, which could expand your lung, causing it to touch the needle.
It sometimes takes 15 minutes or longer to remove the necessary amount of fluid. Most patients feel no discomfort during this time, although a few patients feel some chest pain at the end of the procedure as their lung expands and touches the chest wall. After the fluid is removed, a bandage is placed on your back.
Risk Factors:
This procedure carries a few serious risks, but most patients have no complications. If the needle touches the lung it may create an air leak, which is seen on the x-ray and might require you to stay in the hospital for a few days. Some patients with this complication need to have a plastic tube (called a chest tube) inserted between two ribs. The tube uses vacuum pressure to keep the lung expanded until it has healed.
In most cases, a thoracentesis is performed without complications. Most complications are minor and resolve on their own or are easily treated. Potential complications include the following:
*Pain — Some discomfort may occur when the needle is inserted. Using a local anesthetic helps to reduce the pain. Pain generally resolves once the needle is removed.
*Bleeding — A blood vessel may be nicked as the needle is inserted through the skin and chest wall, causing bleeding. The bleeding is usually minor and stops on its own, although it may cause bruising around the puncture site. In rare cases, bleeding into or around the lung may occur, requiring drainage or surgery.
*Infection — Infection can occur if bacteria are introduced by the needle puncture. Using disinfectant solution to clean the area and using sterile technique during the procedure minimizes this risk.
*Pneumothorax or collapsed lung — Occasionally, the needle used to obtain a fluid sample can puncture the lung. The hole created by the puncture usually seals quickly on its own. If it does not, air can build up around the lung, causing the lung to collapse. This is called a pneumothorax. When a pneumothorax occurs, a chest tube may be used to drain the air and allow the lung to re-expand.
A pneumothorax may also occur if the lung fails to expand when fluid is withdrawn. This is considered to be a drainage-related pneumothorax, and is the most common type of pneumothorax to occur when ultrasound is used for needle placement. Drainage-related pneumothorax is most commonly caused by disorders of the surface lining of the lung and not by the puncture needle. Treatment is rarely needed.
Pneumothorax occurs in less than 12 percent of procedures. Those that do occur are usually small and resolve on their own. A chest tube to helps re-expand the lung is necessary only if the pneumothorax is large, continues to expand, or causes symptoms.
*Liver or spleen puncture — In very rare cases, the liver or spleen may be punctured during thoracentesis. Sitting upright and remaining still during the procedure helps to keep the liver and spleen away from the insertion area and minimizes the risk of this complication.
Must you do anything special after the test is over?
You will need to have an x-ray taken after the sampling is completed. Your breathing should feel the same (or better) after the procedure.
How long is it before the result of the test is known?
The fluid may be tested for a variety of things, including infection and cancer. Cells in the fluid will be examined. It may be several days before full results are available.
Where you may get more information:-Your healthcare provider is the best source of information for questions and concerns related to your medical problem. Because no two patients are exactly alike and recommendations can vary from one person to another, it is important to seek guidance from a provider who is familiar with your individual situation.
This discussion will be updated as needed every four months on our web site (www.uptodate.com/patients). Additional topics as well as selected discussions written for healthcare professionals are also available for those who would like more detailed information.
Some of the most pertinent include:
Professional Level Information:-
Diagnostic thoracentesis
An overview of medical thoracoscopy
Diagnostic evaluation of a pleural effusion in adults
Imaging of pleural effusions in adults
Management of malignant pleural effusions
A number of web sites have information about medical problems and treatments, although it can be difficult to know which sites are reputable. Information provided by the National Institutes of Health, national medical societies and some other well-established organizations are often reliable sources of information, although the frequency with which they are updated is variable.
*American Thoracic Society
(www.thoracic.org)
*American Lung Association
(lungusa.org)
*National Heart Lung & Blood Institute
(www.nhlbi.nih.gov/index.htm)
*National Library of Medicine
(www.nlm.nih.gov/medlineplus/healthtopics.html)
Resources:
https://www.health.harvard.edu/diagnostic-tests/pleural-fluid-sampling.htm
http://www.uptodate.com/patients/content/topic.do?topicKey=~0aPG4xpnulisDf
![Reblog this post [with Zemanta]](https://i0.wp.com/img.zemanta.com/reblog_e.png?w=580)
































